
There is a widespread belief that water from deep aquifers can be eaten without preparation. Indeed, the water is much cleaner than from perched, however, and it has impurities, which can have a negative impact on human health and working equipment. In order to fully understand the issue, contact the specialists of the Department of water purification systems company BIIKS.
Water is a wonderful solvent. Being in constant contact with rocks, it is saturated with the substances of which these rocks consist. Over time, accumulated a huge number of compounds. The composition of the water depends on the type of rock in which the aquifer. For Moscow and Moscow region is characterized by a high content of carbonate hardness salts and compounds of iron.
Prolonged consumption of water of high hardness leads to deposits of stones in the kidneys (stones), in contact skin and hair become dry. While heating the compound to precipitate, forming a solid, bad remove plaque. Deteriorating Heaters, clogged pipes and hoses, increases the rate of wear of the moving parts of the equipment.

Excess rigidity can be determined:
- visually: the formation of plaque on the plumbing and the heating elements in the kettle, heating elements washing machines and dishwashers, boilers);
- taste: compared to bottled water of known hardness;
- for foaming: hard water produces less foam and the consumption of detergents above;
- in the laboratory.
Water softening is the reduction of the concentration of hardness salts and to bring these indicators to the recommended values.
Standards of water hardness
Depending on concentrations of hardness salts, water, divided into:
- soft — salt content of not more than 2 mEq/l;
- normal salt content in the range of 2 — 4 mEq/l;
- hard — salt content in the range of 4 — 6 mEq/l;
- high hardness — salt content higher than 6 mEq/L.
Russian standard governing the quality of drinking water, sets limit value concentrations of hardness salts on the level of 7.0 mEq/L. At that time, as who sets this figure at the level of 2.5 mEq/l, and the EEC adopted the norm of 2.9 mEq/L. Thus, as drinking tap water in Russia, it is acceptable to supply very hard water, with a twofold excess of who recommendations.
Methods of softening of water
It is recommended to soften drinking water and water used for domestic purposes. There are several ways of softening.
Heat
In other words — boiling. With increasing temperature, soluble calcium hydrogen carbonate (the most common compound that triggers stiffness) decomposes to insoluble calcium carbonate and carbon dioxide. The insoluble fraction is precipitated, the gas evaporates. Partially while boiling decreases the concentration of calcium sulfate. The thermal method is the most affordable in the domestic environment, but not the most comfortable and has low productivity. In addition, it is not suitable for compounds of magnesium.
Membrane

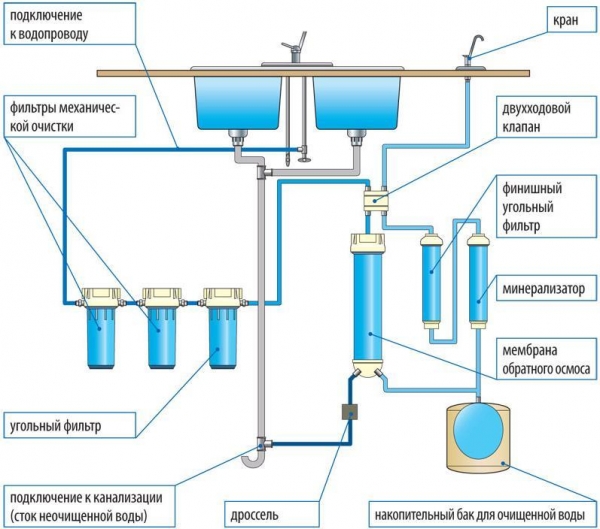
For softening water in this way used molecular membrane that allows only water particles, removing bulk impurities (98%) . So are filters reverse osmosis.
No need to drink contaminated water for the sake of some ostensibly useful salts which it contains. It is much better to nourish your body with the same substances, but in regular products. In fact, humanity all his life and takes them in bread, milk, meat, fish, vegetables and fruit. For example, in a glass of milk one calcium hundreds of times more than in a glass of water. In some cases, for the preparation of drinking water in this way sets the mineralizer.
Chemical (reagent)

The essence of the method is to transform soluble compounds into insoluble. It uses various chemicals depending on the predominance of salts of one type or another. For salts of carbonate type is used lime, sodium compounds, caustic soda and synthetic compounds, e.g., trisodium phosphate. In the end, the water is softened, but due to the presence of the reagents for food use.
Magnetic
Water act by establishing a constant magnetic field. Passage through a magnetic field changes the structure of the hardness salts. Molecules cease to connect when heated and does not form a precipitate, and loosen the existing layer of scale, which is soluble in water. This method does not reduce the concentration of salts and preventing their deposition in the sediment. For domestic purposes, this water well pipes, pumping equipment and heating elements will last longer. Effectively soften water with magnets is possible only in small volumes and flow rates not exceeding 0.5 m/s. using the magnetic water softener also reduces the iron content.
Electromagnetic
An enhanced version of the magnetic with the difference that excess salt not only loses the ability to fall out as sediment, but is removed through a sump into the sewer.
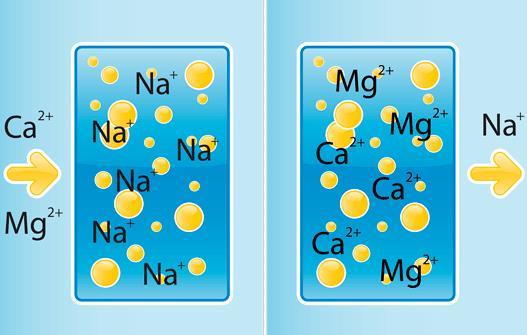
Ion exchange
The method consists in the substitution of calcium ions and magnesium ions sodium compound which is soluble and has no adverse effects on health and equipment.
The modern system of purification of drinking water often combine several methods, which depend on the analysis of water from wells. To determine what type of softener is needed in your situation, will help specialists in water treatment. For artesian wells in the Moscow region, dominated by carbonates, it is recommended to install water softeners are ion exchange type.
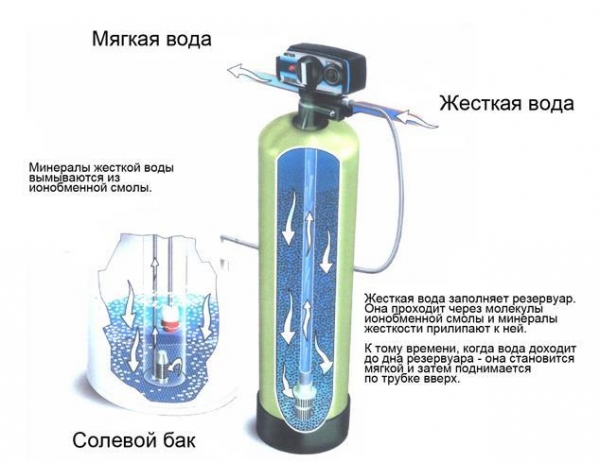
Ion exchange water softener
Structurally, the device is a plastic cylinder inside which in the form of granules is filled with polymeric ion-exchange resin, capable of giving sodium ions and absorb calcium ions and magnesium. The water in the tank, slowly passes through the resin which is the reaction of substitution. When the concentration of sodium ions in the resin drops, to make the process of washing and regeneration. With the container for these purposes is connected to a saline reservoir, where it comes from sodium chloride solution. The process is controlled by an automatic control unit. During flushing, the flow of softened water stops, so the regeneration can be programmed for night time. If the analysis of water occurs continuously, it is recommended to install two containers and start the regeneration alternately. Periodically, on average every 3-4 years, the resin should be changed, as the number of cycles of recovery are limited. System performance depends on the amount of load in the cylinder.